Past News Highlights
 |
New
logo and new website. Our
group is part of the Inserm unit U1212 (CNRS UMR 5320, Univ. Bordeaux)
focused on the natural and artificial regulation of nucleic acids, or 'Acides nucléiques : Régulations Naturelle
et Artificielle' (ARNA). We are happy to report that our unit
has a new logo, and also that we are in the process of completing a new
website. |
|
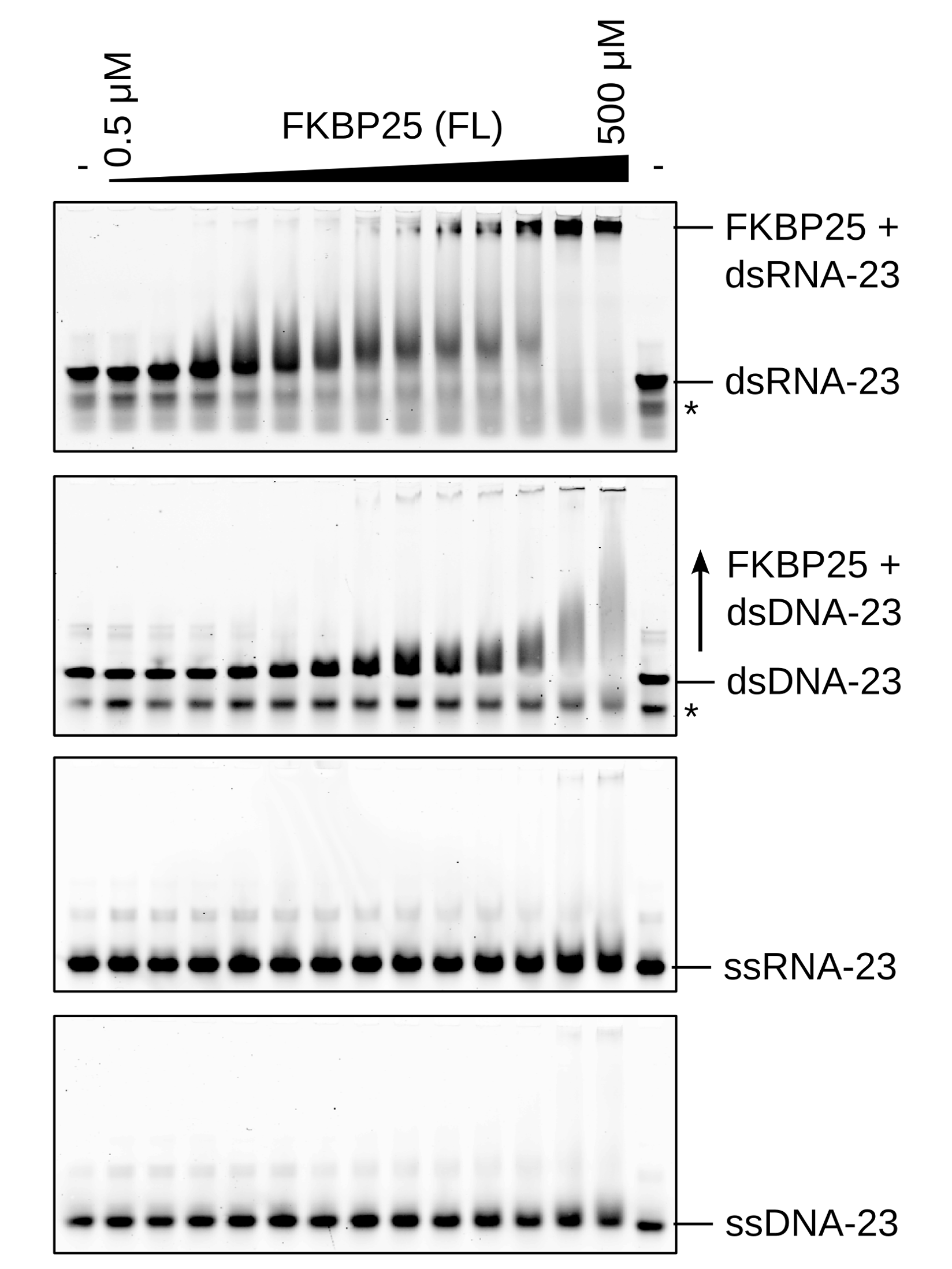 |
A new domain for binding double-stranded
RNA. Recent
work from a long-standing collaboration with the group of Chris Nelson
(Univ. Victoria, Canada) has revealed an RNA-binding behaviour for a
nuclear proline isomerase. Using a combination of proteomics,
microscopy, molecular biology and NMR spectroscopy we have determined
that the N-terminal Basic Tilted Helix Bundle (BTHB) domain of FKBP25
interacts specifically with dsRNA. This association with RNA is
required to mediate interactions with several proteins involved in
ribosome biogenesis. In addition, RNA-binding is essential for the
nucleolar localization of FKBP25. |
Dilworth,
D., Upadhyay,
S.K., Bonnafous, P., Edoo, A.B., Bourbigot, S., Pesek-Jardim, F.,
Gudavicius, G., Serpa, J., Petrotchenko, E., Borchers, C., Nelson,
C.J., Mackereth, C.D.
2017. The basic tilted helix bundle domain of the prolyl isomerase
FKBP25 is a novel double-stranded RNA binding module. Nucleic Acids. Res. 45:11989-12004
|
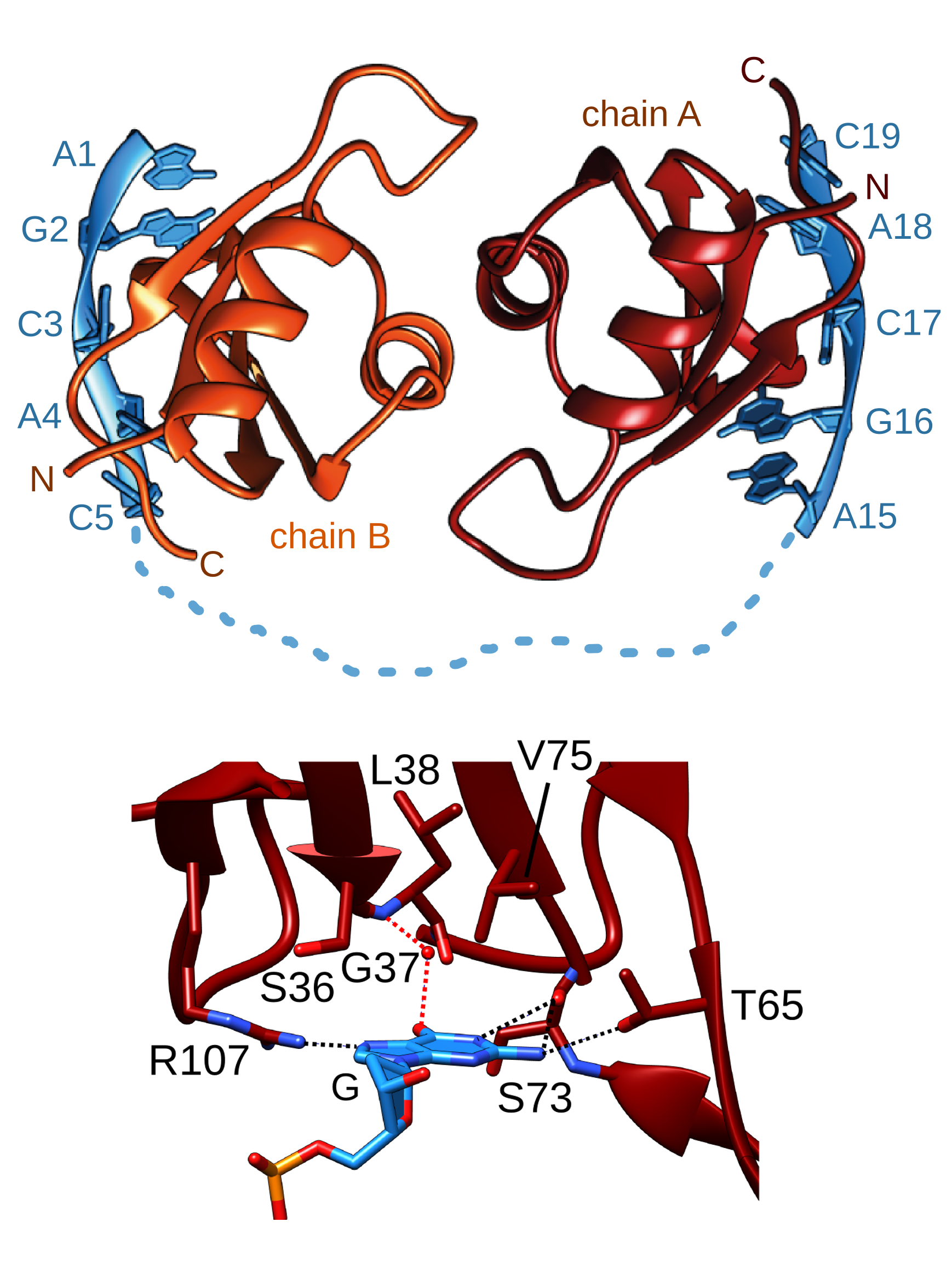 |
The difference that a 'G' makes. The
family of RBPMS proteins (RNA-binding protein with multiple splicing)
all share an RNA-binding Motif (RRM) domain that is required for their
cellular function. Using X-ray crystallography, PhD student Heddy
Soufari has determined the atomic details of the nucleic acid binding
properties of this domain from nematode MEC-8. Using an extensive set
of RNA binding studies with C.
elegans MEC-8, human RBPMS and Drosophila
couch potato, we find that this domain binds tightest to GCAC sequences
in target RNA. The initial 'G' is specifically recognized via several
key hydrogen bonds between the RNA base and the protein. Due to
dimerization, the optimal target is actually two copies of GCAC
separated by at least 6 nucleotides. We are continuing to look at the in vitro and in vivo properties of the
full-length MEC-8 splicing factor. |
| Soufari, H., Mackereth,
C.D. 2017. Conserved binding of GCAC motifs by MEC-8, couch potato and
the RBPMS protein family. RNA
23:308-316. |
 |
Chemical biology: studying
the interaction between a synthetic quinoline-based foldamer and
human carbonic anhydrase. In collaboration with the team of
Ivan Huc, we have looked at the solution behaviour of a
foldamer–protein complex by NMR and circular dichroism. Inspired by the
atomic details of the previous crystal structure we looked specifically
at foldamer-mediated protein dimerization and the process of foldamer
handedness induction. Looking at this hybrid complex in
solution gives us clues as to how to design future foldamers to
specifically interact with proteins. More to follow soon! |
Jewginski,
M., Fischer, L., Colombo, C., Huc, I., Mackereth, C.D. 2016. Solution
observation of dimerization and helix handedness induction in a human
carbonic anhydrase-helical aromatic amide foldamer complex. ChemBioChem 17:727-736.
|
 |
A new trick for oligoureas. We have
added our expertise in solution NMR spectroscopy to work led by the
team of Gilles Guichard. The goal was to help in the
characteization of a series of oligoureas that with a small change in
sequence can exist as either a hexamer bundle or an extended channel.
The extra properties of pH-sensitivity and, for the hexamer, a central
hydrophobic cavity suggest that these oligoureas will have many
interesting future uses. |
Collie,
G.W.,
Pulka-Ziach, K., Lombardo, C.M., Fremaux, J., Rosu, F., Decossas, M.,
Mauran, L., Lambert, O., Gabelica, V., Mackereth, C.D., Guichard, G.
2015. Shaping quaternary assemblies of water-soluble non-peptide
helical foldamers by sequence manipulation. Nat. Chem. 7:871-878.
|
|
Sugars such as
glucose, fructose, mannose or galactose exist in different forms and
are particularly difficult to discriminate. The high selectivity of a
specific foldamer towards fructose thus seems to be a promising
solution regarding such an issue. Designed from modular artificial
strands and able to fold into well-defined conformations, foldamers may
form cavities complementary to small molecules such as monosaccharides.
The highly predictable structure of these artificial molecules is a
clear advantage in creating new receptors.
Read more from IECB
|
Chandramouli,
N.,
Ferrand, Y., Lautrette, G., Kauffmann, B., Mackereth, C.D., Laguerre,
M., Dubreuil, D., Huc, I. 2015. Iterative design of a helically folded
aromatic oligoamide sequence for the selective encapsulation of
fructose. Nat. Chem.,
dor:10.1038/nchem.219512.
|

|
 |
A short commentary on the atomic complexity that forms the basis of
functional interactions between proteins. In this case, the highlight
is on the interaction between the splicing factors SUP-12 and ASD-1,
and our data from the 2014 article in Nature Communcations.
|
Mackereth,
C.D. 2014. Splicing factor SUP-12 and the molecular complexity of
apparent cooperativity. Worm.
3:e991240
(open access)
|
 |
 |
In collaboration with the team of Denis Dupuy we have looked into the
atomic details of RNA-binding for the alternative splicing SUP-12. This
protein helps to regulte muscle-specific alternative splicing in the
worm Caenorhabditis elegans,
and we have determined the structure of the RNA-binding domain bound to
a high affinity ligand G-G-U-G-U-G-C, by using NMR spectroscopy.The
atomic details were used to make mutations in fluorescent mini-gene
reporters, in order to translate the in
vitro
findings to observations in live worms. We have also investigated the
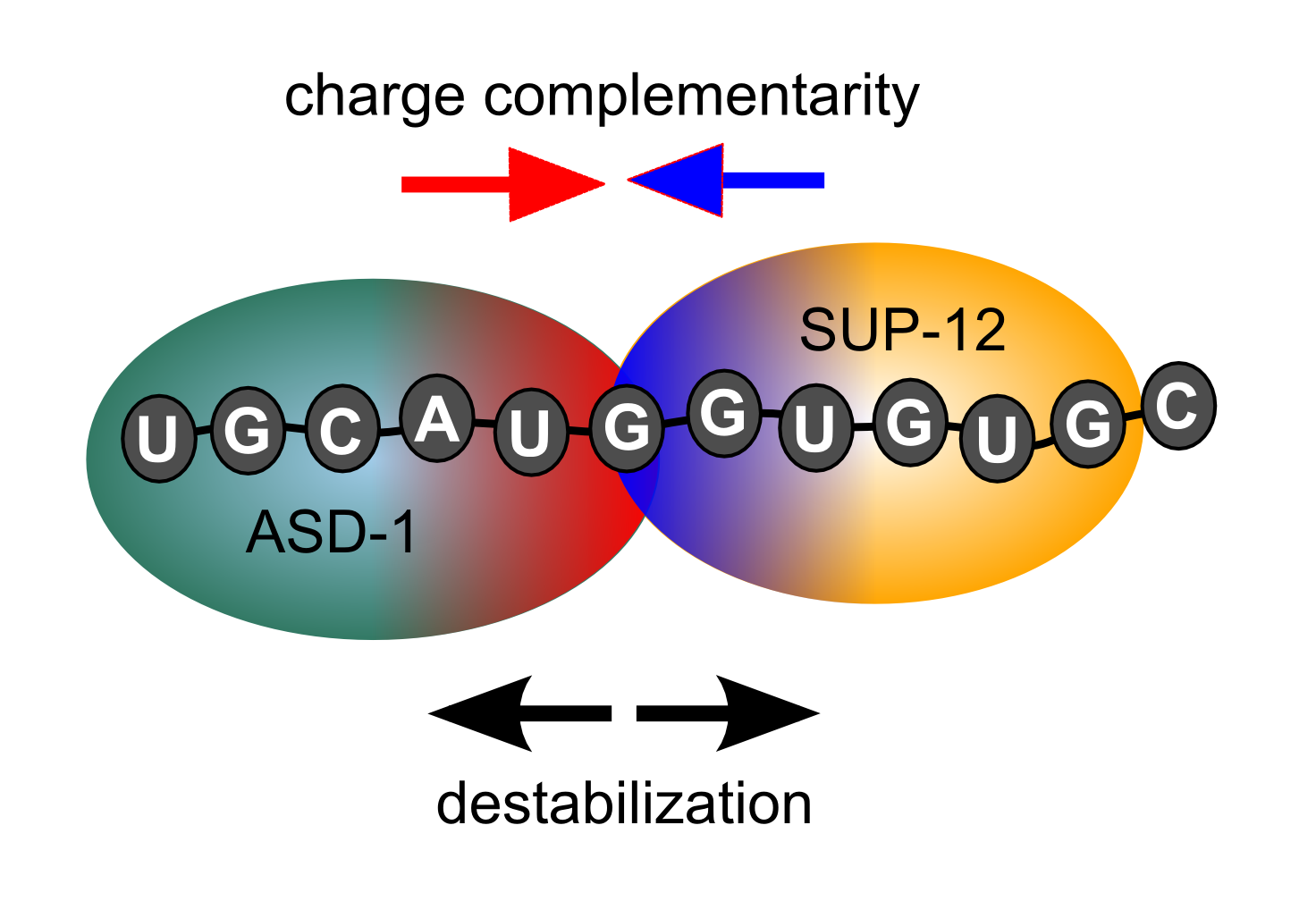
interaction of SUP-12 with another alternative splciing factor, ASD-1.
We found that ASD-1 directly contacts SUP-12 on the RNA but does not
increase its affinity for RNA.
|
| Amrane, S., Rebora, K.,
Zniber, I., Dupuy, D., Mackereth, C.D. 2014. Backbone-independent
nucleic
acid binding by splicing factor SUP-12 reveals key aspects of molecular
recognition. Nat. Commun. 5:4595. |
|
 |
|
 |
Shape-shifting mechanism in the
regulation of human genes
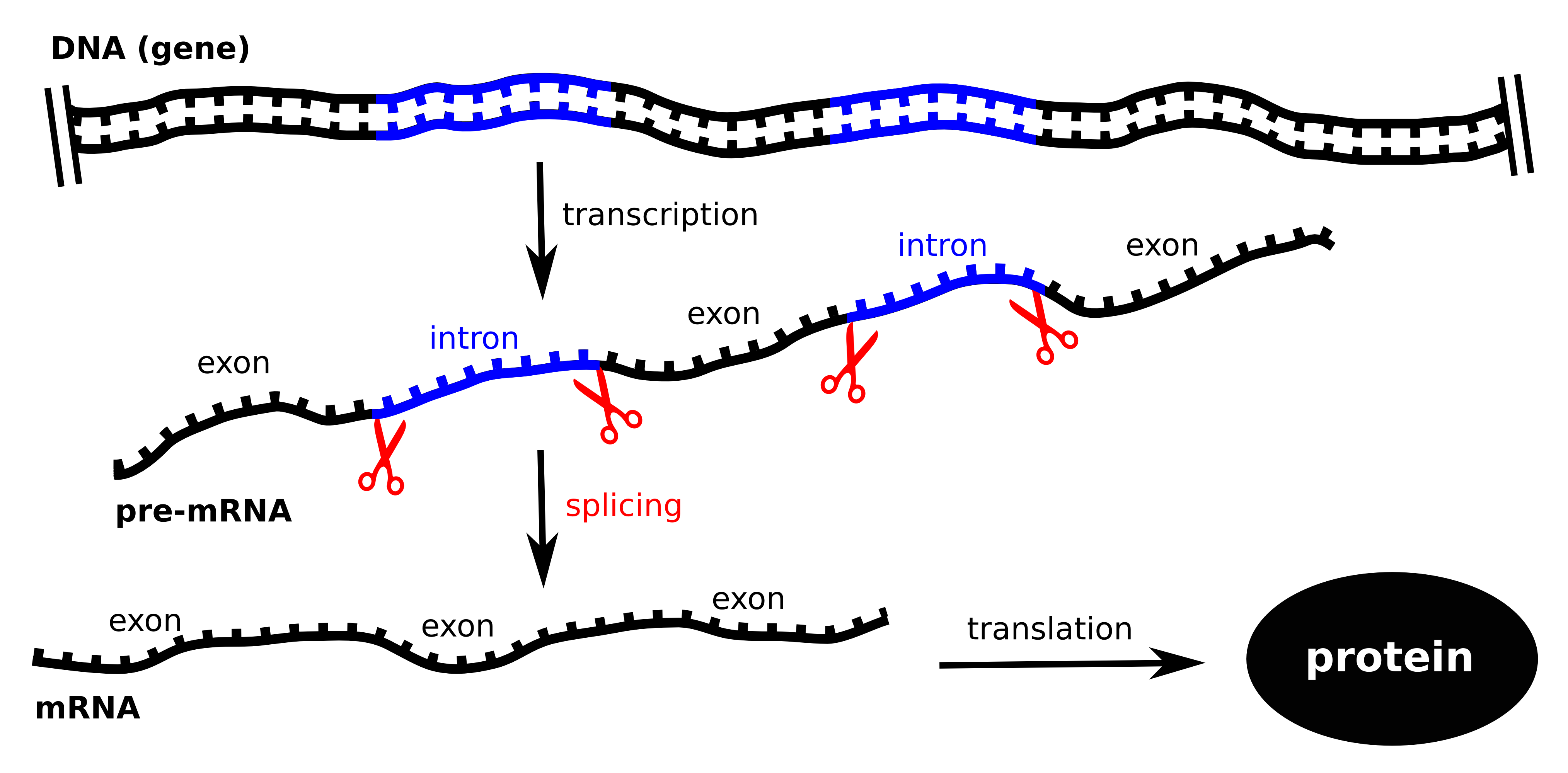
In
order to create proteins, the protein-coding gene must be transcribed
into RNA and in the so-called splicing process interrupting segments
are removed to form the correct protein-building instructions. Along
with scientists
at the Helmholtz Zentrum München and the Technical University of
Munich (TUM), the European Molecular Biology Laboratory (EMBL) in
Heidelberg and the Centre for Genomic Regulation in Barcelona, we have
now
discovered how the human U2AF protein enables this process. The
results have been published
in the July 21 issue of Nature.
|
The genes in the human genome have a specific structure. Sections with
relevant exons alternate with areas known as introns, which contain
irrelevant information. In order for a protein to be created, the
pre-messenger RNA (pre-mRNA) first has to be copied from the DNA. The
copy is then spliced and the introns are removed, leaving only the
mRNA; which consists solely of exons. For this purpose, the introns
must be recognized and accurately excised. This process is also known
as the central dogma of molecular biology: genetic information only
flows in one direction: from the DNA to RNA to proteins. |
 |
|
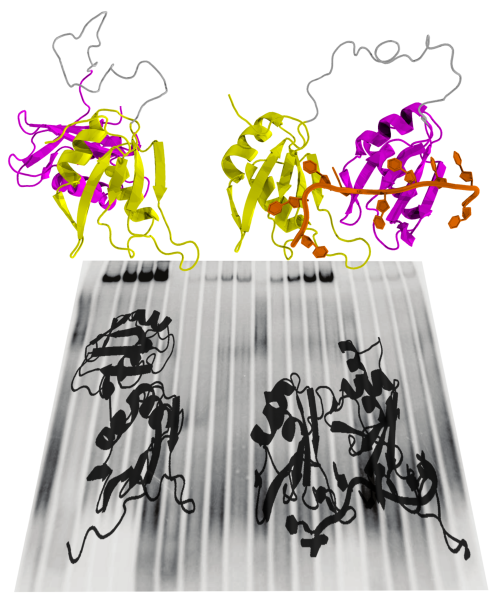
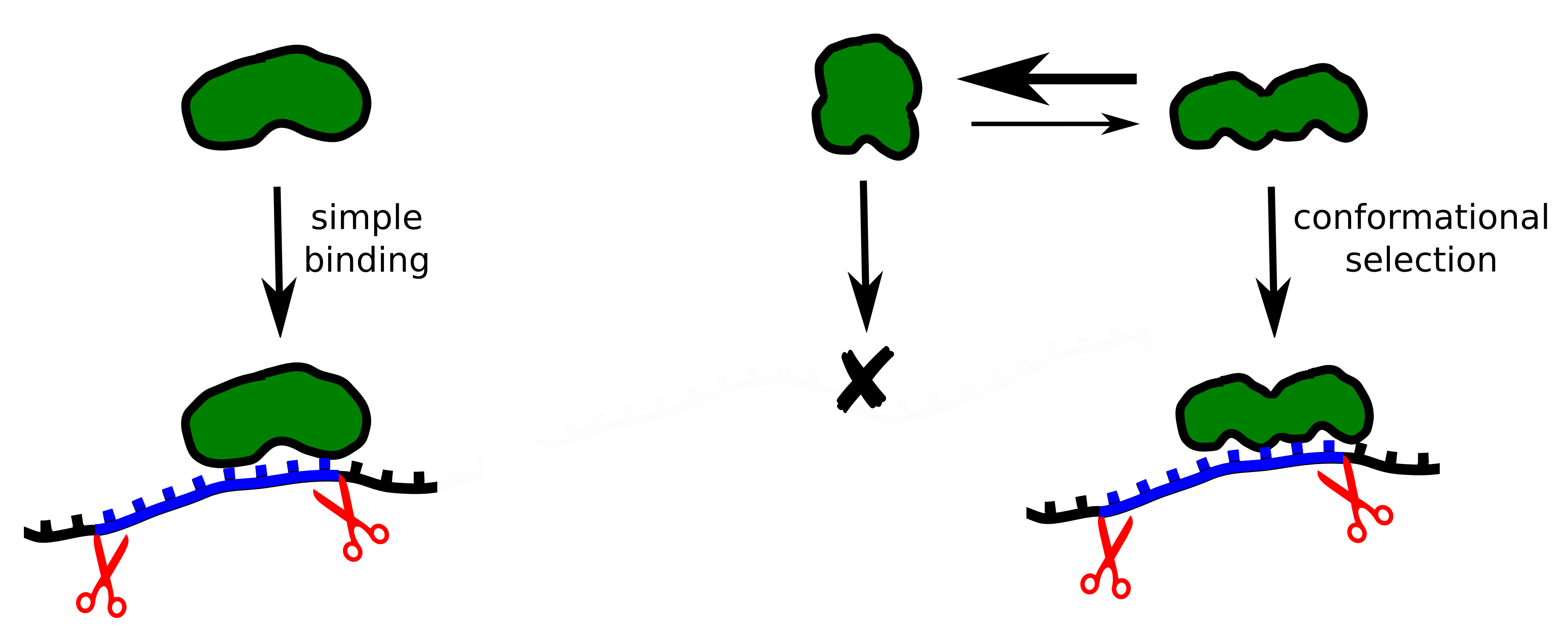
Splicing
requires the cooperation of different proteins, or splicing factors.
One such splicing factor, U2AF, was examined in collaboration with
German
and Spanish scientists. It consists of two structural units and binds
to the RNA close to the intron-exon interface. The spatial
structure of the U2AF protein alternates between a closed and an open
conformation. A matching RNA sequence in the intron causes the U2AF
to favour an open conformation, which activates splicing and leads to
the elimination of the intron. The intron’s RNA sequence determines
how effectively this conformational change can be stabilized. This
shift of balance between the closed and the open form of the U2AF
protein occurs through a process of natural selection. we
presume that similar shape-shifting mechanisms – balanced between a
closed, inactive and an open, active conformation – play an
important role in the regulation of many other signal pathways in the
cell.
|
 |
| Mackereth,
C.D., Madl, T., Bonnal, S., Simon, B., Zanier, K., Gasch, A., Rybin,
V., Valcarcel, J., Sattler, M.
2011. Multi-domain conformational selection underlies pre-mRNA splicing
regulation by U2AF. Nature 475:
408-411. |
 |
 |
 |
Also reported in :
Le
Quotidien du médecin
SpectroscopyNOW
 |
We have
recently investigated a tight heterodimer
formed between two proteins from a yeast Saccharomyces
cerevisiae complex involved in the 3' processing of
pre-mRNA. The cleavage/polyadenylation factor IA (CF IA) complex is
composed of four proteins (Clp1p, Pcf11p, Rna14p, Rna15p) that
recognize RNA sequences adjacent to the cleavage site and recruit
additional processing factors. We have solved the solution structure of
the tether complex composed of the interacting regions between Rna14p
and Rna15p.
The
C-terminal monkeytail domain from Rna14p and the hinge region from
Rna15p display a coupled binding and folding mechanism, where both
peptides are initially disordered. We have used the structure to
understand the molecular basis of temperature-sensitive mutations and
find that the main consequence is the loss of Rna15p (and its important
RNA-binding domain) from CF IA. This tight association complex is not
just important for yeast: conservation of interdomain residues reveals
that the structural tethering is preserved in the homologous mammalian
cleavage stimulation factor (CstF)-77 and CstF-64 proteins of the CstF
complex. |
Joining the
efforts of IECB colleague Sébastien Fribourg and his laboratory,
we have helped to reveal the structure and dimerization of the
N-terminal domain of Drosophila
CstF-50. This small domain, composed of three alpha-helices, forms a
homodimer both in the crystal
structure
and in solution. The protein is a component of the Cleavage Stimulation
Factor (CstF), a complex that is a critical part of the pre-mRNA 3'
processing machinery, and is required for accurate production of the
mature mRNA poly(A)
tail. CstF consists of the three subunits CstF-50, CstF-64
and CstF-77. Along with the dimerization
of CstF-77,
the dimer function of the domain strengthens an overall stoichiometry
of two copies of each subunit in the complex, creating a hexameric
CstF assembly.
***************************************************************************************************
|

